The Hidden Costs of Reliability Failures
Is your reliability bathtub full of hidden quality management costs?
In a perfect world, every medical device would benefit from substantial reliability assurance.
Testing, demonstration, analysis, and prediction allow management to make informed decisions about their devices. In reality, every medical device company has restrictions that limit test coverage and prototypes because limited budgets, time, personnel, and more.
Before release, a new device may only have the minimum amount of testing required to meet regulatory standards. That’s why newly-released medical devices often lead to significantly higher lifetime reliability expenses than anticipated. Hidden failures can arise from software. Software doesn’t wear out but does have an increasing failure rate over time. The entire design including hardware, software, environment, and human factors affects reliability and costs.
HOW THE BATHTUB CURVE FILLS UP WITH FAILURES
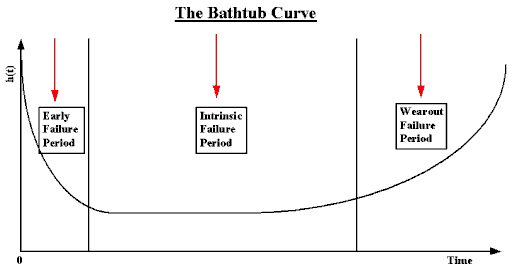
When you look at device reliability data, it often forms a bathtub curve – a statistical model that shows reliability results. Over time, your device failure rate descends, plateaus, and ascends again. This represents the lifecycle of the medical device. Over time hidden failures are revealed and fill up ‘the bathtub’.
In its early life, the device’s failure rate is high, but this rate rapidly decreases as defects are identified and remedied. This happens during early adoption of the device. Then, in its mid-life, the failure rate is lower and becomes more constant over a long period. This is the steady-state period. Later, as its end-of-life-expectancy approaches, its failure rate spikes back up again due to wear and tear. This is the repair or replace period.
Is there anything medical device companies can do to mitigate the impact of the bathtub curve? Absolutely!
They can adopt expert-level quality management practices for their medical devices. This includes predicting potential reliability failures and related costs, then minimizing them throughout the product lifecycle.
EXAMPLES OF RELIABILITY FAILURES AND ASSOCIATED COSTS
As medical devices age, their costs quickly fill up the bathtub. To fully capture the breadth of reliability costs in quality management, all activities related to product reliability must be included. A ten-to-one rule is generally accepted to indicate rising costs as the design evolves from concept, input, output, review, verification, validation, and release stages. A ten- or hundred-dollar problem could be recognized and fixed early; if not, potentially a million-dollar problem results after release if this involves serious mitigations.
Below is a chart that shows common reliability activities for medical devices and the potential costs associated with each.
Reliability Activity Typical Cost per Incident
Surveillance documents………………………….$200
Rework…………………………………………………………….$300
Complaints…………………………………………………….$500
CAPA………………………………………………………………..$2500
Failure analysis…………………………………………….$5,000
Field action……………………………………………………$Millions
Reputation…………………………………………………….$Millions
Lost opportunity cost………………………………..$Millions
Some costs are more obvious than others from the outset of the process. The most damaging expenses are often hidden from immediate view. For example, consider the enormous cost of damage to the company’s reputation. This isn’t initially obvious at the beginning of the bathtub curve when reliability failures are starting to drop and level off. But by the time the curve is rising again toward the end of the device lifecycle, the company could be suffering bad publicity that ultimately costs millions of dollars in lost business.
Another hidden cost of product reliability is the increased expense of delaying product reliability improvements. This involves the relative cost of a single design improvement for a medical device as a function of the product’s lifecycle, which is broken down in the chart below.
Life Cycle Phase Cost of Change (Relative)
Surveillance documents………………………..$10
Rework………………………………………………………….$100
Complaints………………………………………………….$1,000
CAPA………………………………………………………………$10,000
Failure Analysis………………………………………….$10,000
Reliability is a lagging indicator of design quality; reliability costs are a lagging (financial) risk. Companies often have “warranty reserve” type financial accounts and “customer goodwill” type of financial accounts to help in these matters. These are hidden costs of doing business.
These types of costs justify early intervention in quality management to prevent the buildup of expenses due to reliability failures. Unfortunately, these costs can continue to escalate well after a product is first introduced.
HIDDEN COSTS CONTINUE TO ACCUMULATE AFTER SHIPPING
Even after the product is shipped to the customer, costs continue to rise. Cascading like dominoes, one cost leads to another. The total lifetime cost of these reliability failures can be astronomical.
Even after device release, when the product is shipped to the customer base, costs continue to rise. A stream of unreliability issues starts to fill the bathtub. Many of these would have been overlooked or inadequately addressed without dedicated resources. The total lifetime cost of these reliability failures can be astronomical.
Reliability engineering intervenes to minimize hidden or under-appreciated reliability risks. Each halted reliability failure prevents another failure from ever happening.
Over time, these costs and failure become more minimal and predictable. This aspect of quality management is crucial for a medical device manufacturer’s long-term financial stability and competitive strength.
Hidden costs can be detrimental towards the success of a company. That is why quality management success requires talented reliability engineers who can oversee the process and ensure things go smoothly.
The ideal reliability engineer has the following attributes:
- Is a subject matter expert
- Is ASQ certified
- Shows dedication to the principles, practices, and procedures of reliability engineering
- Has a minimum of 10 years of medical device reliability engineering expertise
The reliability engineers at Network Partners meet all the criteria above, plus we use world-class data analysis to provide deep insights into your overall quality management process and reliability practices. We collaborate with our clients to establish reliability targets, reliability growth, and the body of evidence for reliable product performance to manage lifecycle costs.
HOW TO DRAIN THE BATHTUB AND KEEP IT CLEAN
The best way to drain the bathtub is to prevent it from filling up with reliability failures in the first place. Network Partners helps control hidden reliability costs, especially when you engage with us early in your product’s lifecycle.
Reach out to Network Partners today to create a quality management plan for tomorrow. We provide the medical device expertise you need to minimize expenses while bringing life-saving products to the people who need them.