Save the Date for Your Label!
How often do you look at your medical device products’ barcodes? Do you know that each number means something? Do you know that you’re presenting information that needs to be truthful and accurate?
Regulatory labeling isn’t as notable as getting market clearance but it’s very important and necessary to get your product to market and to keep it there. This industry utilizes barcode labeling for many different applications and if it’s not done correctly, you could cause your team noncompliance issues, tracking issues, and headaches to say the least.
GS1 recently introduced a new requirement that we all need to get behind and it affects you and your barcodes.
Dates on the Label
There are two areas on the label where the date is indicated. The first is on the body of the label typically accompanied by the ISO symbol for the expiration or date of manufacture.
The second location of the date appears in the automatic identification and data collection (AIDC) or what is better known as the linear barcode or 2D datamatrix code.

Current State
How a date is indicated on the body of a label may differ based on the geography in which it is sold. For instance, in the EU under MDR, the date must be expressed in at least the year and month. In the US, the FDA has mandated that the date format follows the year, month format, or YYYY-MM-DD.
Because there are different regulatory requirements that appear on the body of a label, there may be times when it is not necessary to specify the exact day on the label. For this reason, GS1 currently allows a manufacturer to populate the day of the month using two zeroes (00). This is to be interpreted by the user as the last day of the noted month.
Future State
GS1 recently updated their general specification to make an actual day of the month required within the data encoded. Starting on January 1, 2025, GS1 will no longer recognize two zeroes (00) within the encoded data or the human readable interpretation; a valid day of the month must be included in the AIDC.
Where will we see this being impacted?
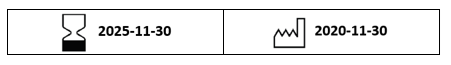
Any label that contains a Production date and/or Expiration date within the AIDC and the date portion is currently populated with two zeroes. See example below:
What does this mean to you and what do you need to discuss with your team?
- Awareness of the new date requirements and get your team talking about what this means to your current practice
- Acknowledge the implementation and start working backwards to determine when you need to kick this effort off
- Identify how this new data will impact your systems and inputs

We don’t always love change but when you can plan for it, it’s so much easier to implement! Happy labeling.
For more information see GS1 General Specifications.
Written by Heather Valley, RAC, Senior Director of Regulatory Affairs at Network Partners and Michelle Oliveira, Associate Director of Regulatory Labeling at Network Partners.



