EU MDR Extended Transitional Period
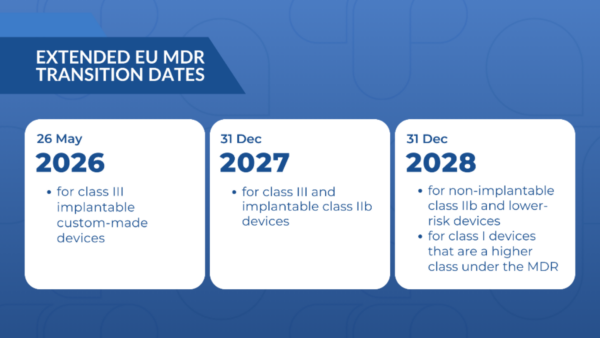
Figure 1 – Extended Transition Dates
On March 15, 2023, regulation (EU) 2023/607 amended the Medical Device Regulation (MDR) and the In Vitro Diagnostic Medical Devices Regulation (IVDR) to ensure patient safety and prevent shortages of critical medical devices. This amendment gives manufacturers and notified bodies more time to perform conformity assessments as per the MDR for devices certified under Directive 90/385/EEC or Directive 93/42/EEC.
The new transitional periods, which now end either on December 31, 2027, or December 31, 2028, depending on the device’s risk class as per the MDR’s classification rules (Annex VIII), as seen in Figure 1, offer significant flexibility. The risk class might differ from the one indicated on the original certificate, influencing the applicable MDR requirements during the transitional period. For the full timeline, see Figure 2. This flexibility empowers manufacturers and notified bodies to adapt their strategies and timelines accordingly.
Devices already certified under the MDR can also benefit from the extended transitional period, provided their MDD/AIMDD certificates have not been withdrawn by the notified body. The certification under MDR itself does not necessitate the withdrawal of MDD/AIMDD certificates, meaning both legacy and MDR-compliant devices can coexist in the market until the end of the transitional period.
How Network Partners Group Can Help
At Network Partners Group (NPG), we understand the complexities of navigating regulatory changes. Our team of experts is dedicated to helping your company meet the MDR compliance deadlines effectively. We offer:
- Regulatory: Expertise in providing regulatory EU MDR compliance, from gap assessments to guiding clients through the intricate landscape, ensuring seamless transitions and full adherence to the new regulations.
- Quality: Ensuring high quality standards in medical devices are met through quality management systems that are aligned to the stringent requirements of the EU MDR.
- Labeling: Accurately revise thousands of labels in your system, provide objective evidence of verification, and speed label approval in your change control system. Our team can also validate your label content.
- Packaging: Expertise in ensuring the product packaging meets the state-of-the-art EU MDR requirements, from gap assessment through full remediation to guarantee full compliance.
- Project Management: Effective project management involves creating a step-by-step process to execute EU MDR remediation requirements across all cross-functional workstreams impacted and documenting the newly created workflows. Our team can robustly manage the end-to-end implementation of EU MDR submission responses to notified bodies and track the deficiency questions and responses being closed out in a timely manner.
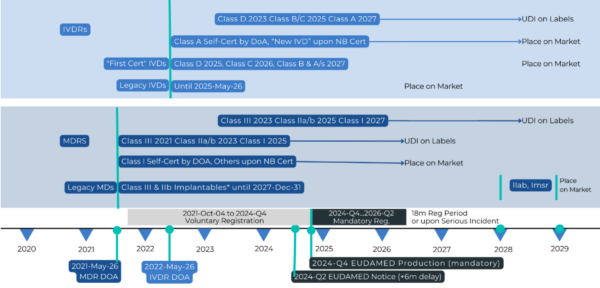
Figure 2 – Extended EU MDR Timeline
Let NPG guide you through these regulatory changes, ensuring you confidently meet the deadlines. Contact NPG today to ensure your devices stay compliant and continue to thrive in the market.



